The ODAC Dermatology, Aesthetic & Surgical Conference (ODAC) recently announced changes to its advisory committee. Dermatologist Adam Friedman, MD, will now serve as ODAC co-chair. Dermatologist and Mohs surgeon James Spencer, MD, will now serve as ODAC co-chair emeritus.
Dr. Friedman, who has served as ODAC medical director since 2017, will now oversee ODAC’s medical dermatology offerings. He will serve alongside dermatologists and Mohs surgeons Joel Cohen, MD, who is co-chair of procedural dermatology, and Susan Weinkle, MD, who is co-chair of aesthetic dermatology. Dr. Friedman also is chair of the GW SMHS Department of Dermatology in Washington, D.C., and is the founding director of the GW Dermatology Residency Program, director of Translational Research in the Department of Dermatology and director of the Supportive Oncodermatology Program at the GW Cancer Center.
“Dr. Friedman has a wealth of knowledge on both medical dermatology and medical education, including how to create compelling educational experiences that translate into improved patient care,” said Shelley Tanner, CEO and president of SanovaWorks, which produces ODAC. “We look forward to his continued contributions for years to come.”
Dr. Spencer, who has served as ODAC chair for 10 years, will now serve as a senior advisor. He will also continue lecturing at ODAC on skin cancer related topics. Dr. Spencer is in private practice in St. Petersburg, Fla., and also serves as clinical professor of dermatology at The Mount Sinai School of Medicine in New York.
“Dr. Spencer has been instrumental in ODAC’s growth since the conference’s inception,” said Ms. Tanner. “We are grateful for his leadership and look forward to his continued impact through his advisory role.”
ODAC, which was founded in 2003, historically attracts nearly 800 dermatology physicians and allied health professionals each year. The 2022 event will take place January 14-17 at the Rosen Shingle Creek in Orlando.

New York (Mar. 17, 2021) – Adam Friedman, MD, medical director of the ODAC Dermatology, Aesthetic & Surgical Conference (ODAC) was recently named chair of the GW SMHS Department of Dermatology in Washington, D.C. Dr. Friedman has served as interim chair since February 2019, and is the founding director of the GW Dermatology Residency Program, director of Translational Research in the Department of Dermatology and director of the Supportive Oncodermatology Program at the GW Cancer Center.
During his time as interim chair, Dr. Friedman further strengthened collaborative ties between ODAC and GW SMHS through multiple and ongoing educational activities, including the GW Translational Science Lecture Series, the award-winning Krazy Kodachromes, and more recently, the 2020 DCAD conference, the GW Appraisal of Acne conference and the Core Crusher Basic Boot Camp Exam Prep Course.
“Dr. Friedman has worked tirelessly to ensure all dermatologists have access to the latest educational developments in the specialty,” said Shelley Tanner, CEO and president of SanovaWorks, which produces ODAC. “He expertly knows how to engage audiences in their education, creating programs that make learning compelling and applicable to daily practice. We are thankful to have his insight at work in our programs.”
Dr. Friedman has served as ODAC medical director since 2017. In this role, Dr. Friedman has been instrumental in cultivating and expanding the medical dermatology section of the educational program, in addition to generating opportunities for residents.
Dr. Friedman also serves on the editorial board of the Journal of Drugs in Dermatology (JDD), hosts the JDD Podcast and is the senior editor of and chairs the Derm In-Review Advisory Council. JDD and Derm In-Review are also products of SanovaWorks.
ODAC, which began in 2003, historically attracts nearly 700 dermatology physicians, residents, nurse practitioners and physician assistants each year. The 2022 event will take place January 14-17 at the Rosen Shingle Creek in Orlando.
###
Source: Next Steps in Derm
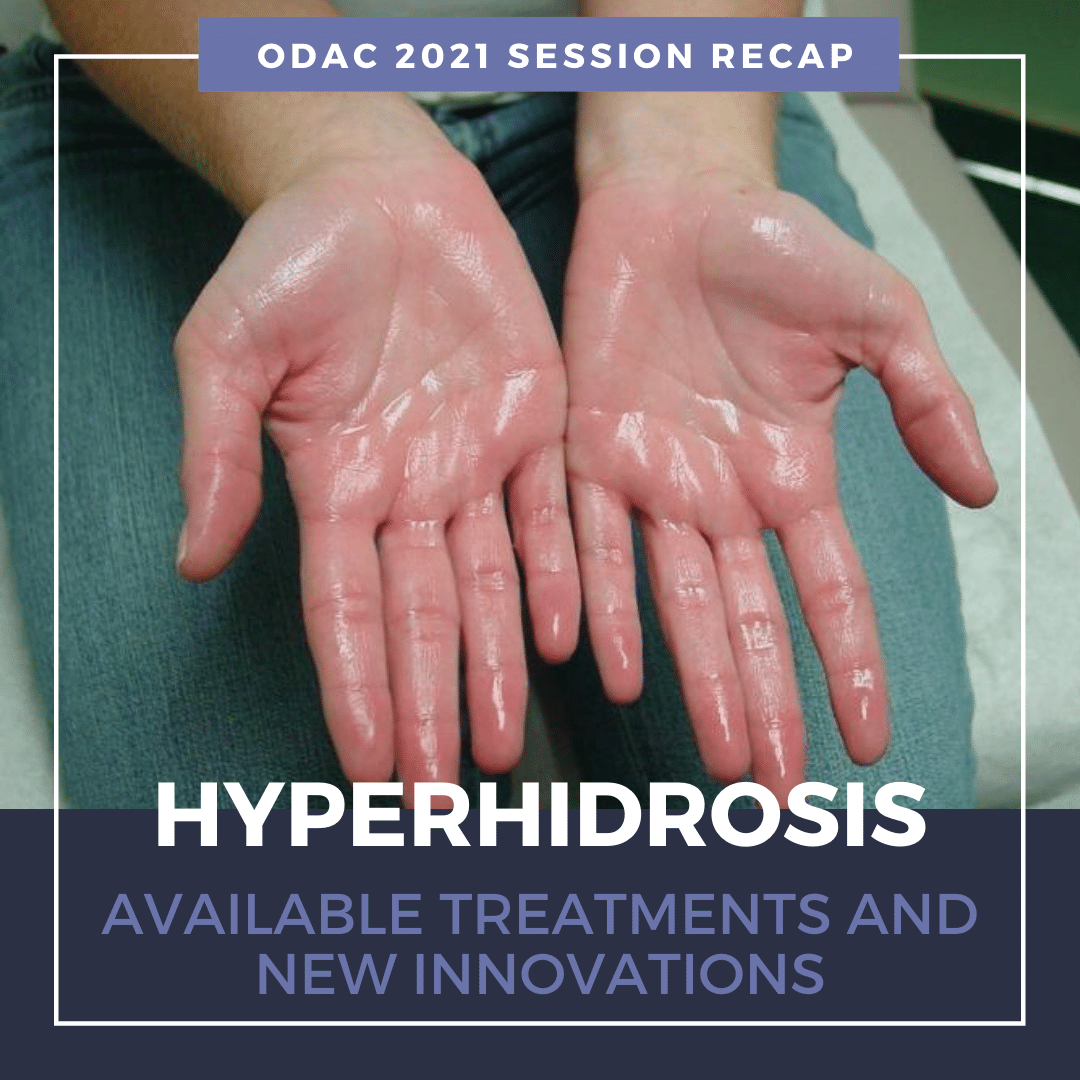
What is Hyperhidrosis (HH)?
Primary HH is defined as excessive, bilateral, and generally symmetrical sweating at abnormal levels unprompted by activity or environment.
Hyperhidrosis is characterized by abnormal sweating beyond what is needed for thermoregulation. It is not a normal physiologic response. Nearly 15 million people in the United States suffer from hyperhidrosis and half of those individuals have axillary hyperhidrosis. HH peaks in adulthood and approximately two-thirds of these individuals have never discussed their primary axillary hyperhidrosis with a healthcare professional.
Why aren’t more patients seeking help for their abnormally excessive sweating?
Many of these patients do not realize their excessive sweating is a medical condition and are unaware of protentional treatment options. On average, patients wait at least 10 years before seeking medical help for their abnormally excessive sweating1.
Patients with hyperhidrosis are reluctant to seek medical care, despite the negative impact on their quality of life.
Primary versus Secondary Hyperhidrosis
Primary Focal Hyperhidrosis is defined as excessive focal sweating of at least 6 months duration without any obvious cause with at least two of the following features:
-
- Onset before age 25, often as early as 9 years old
- A bilateral and symmetric pattern
- Impairment of daily activities
- Occurring at least once per week
- Cessation during sleep
- A positive family history
90% of all diagnosed cases of HH are primary and two-thirds of all HH patients report a positive family history.
Secondary Generalized Hyperhidrosis can be caused by or associated with another medical condition or results as a side effect of a medication. It usually presents with large areas of the body affected. Patients with secondary HH may experience sweating even when they are sleeping. It is important to differentiate between primary and secondary hyperhidrosis. Review the patient’s medical history and review of systems should point to any secondary causes. Patients who do not fit the classic pattern of primary hyperhidrosis should undergo further evaluation, and may need laboratory studies or radiographic evaluation.
Can the coronavirus spread through sweat?
There is no evidence that Covid-19 can be transmitted through sweat. The virus is transmitted by respiratory droplets. Dr. Adam Friedman notes that sweat could actually help prevent the transmission of Covid-19 due to the inherent antimicrobial activity of sweat. For anyone worried about transmission of the virus, it’s important to emphasize key tenets voiced from experts:
-
- Wash/sanitize your hands often and well
- Avoid touching your face
- Wear a mask in public
- Maintain physical distance, about 6 feet, from others
- Sanitize frequently-touched surfaces
- Follow your community’s public health recommendations
For more information, visit the International Hyperhidrosis Society’s (IHS) website: www.sweathelp.org
IHS is the only independent, non-profit, global organization that strives to improve quality of life among those affected by hyperhidrosis.
Treatment of Hyperhidrosis
A wide range of techniques has been utilized in the treatment of HH. First-line therapy for HH is topical formulations. Over-the-counter antiperspirants are usually not strong enough to significantly improve excessive sweating. Prescription aluminum salts are usually the first-line treatment for HH. The aluminum salts act as a plug, precipitating and blocking the sweat ducts. Sweating is reduced in the evening. Thus, application to dry body surfaces at night is ideal. Non-medicated deodorant can be applied in the morning after bathing. The fragrance from deodorants can be used to mask body odor from the microbiome of the skin.
Topical Aluminum Pearls:
-
- Aluminum salts must remain on the skin for 6 – 8 hours to be effective
- Overnight application is recommended
- Wash treated skin in the morning before sweating begins
- Wait 24 to 48 hours after shaving
- Beware of damage to fabrics
- A reaction occurs when aluminum mixes with the salts of your sweat, which may cause yellow stains on white fabrics.
- Irritated skin can be treated with low potency topical steroid
- Aluminum salts must remain on the skin for 6 – 8 hours to be effective
Topical antiperspirants are just not that effective for hyperhidrosis.
While over-the-counter products are most commonly recommended, they offer the least patient satisfaction. Topical antiperspirants are safe and simple to use. However, application is time-consuming, only provides temporary relief, and may cause skin irritation at the application site.
Fortunately, there are novel topical therapies available to patients with excessive underarm sweating, or axillary hyperhidrosis. Topical glycopyrronium tosylate (GT) wipes are FDA approved for treatment of axillary HH. This is the first and only prescription cloth towelette approved to treat excessive underarm sweating. GT works by blocking receptors responsible for sweat gland activation1. A significant number of patients can see improvement as early as 1 week. After 4 weeks of treatment in the clinical trials, GT resulted in substantially greater improvements compared to the control group. Patients were happy with the fast onset of effect and reported overall improved quality of life2:
-
- No longer avoiding interactions with other people
- No longer showering/bathing more than once a day or changing shirts during the day
- Feeling less embarrassed or confident
- No longer avoiding activities for work or hobby
Topical Glycopyrronium Tosylate Pearls:
-
- Approved for use in children ≥9 and adults
- Applied nightly to clean axillary skin
- Use 1 towelette by wiping across each entire underarm once
- Wash hands thoroughly after use to avoid inadvertent transfer of the drug to areas such as the eyes
- No need to remove hair or occlude the area for use
- May use OTC antiperspirants as needed
- Most patients see improvement by 2-3 weeks
- May hold dose as needed
Dr. Friedman notes that use of topical GT for treatment of palmar/plantar hyperhidrosis is off-label. He instructs his patients to rub the towelette between hands until dry, apply Aquaphor, and then wear gloves for at least 1 hour, in order to achieve clinical benefits.
It is important to monitor for anticholinergic side effects. The most common reported adverse effects include dry mouth and mydriasis. Use of gloves with the towelette and careful handwashing can help mitigate anticholinergic side effects. GT is non-invasive, effective, and well-tolerated. There is minimal irritation and can be safely used in pediatric populations. Aside from long-term therapy and potential for cost issues, topical GT provides clinically meaningful benefits for patients with primary axillary hyperhidrosis.
Iontophoresis
Iontophoresis is a process of transdermal drug delivery by use of a voltage gradient on the skin. It is often recommended for patients who have failed topical therapies or in patients with HH of the palms and soles. During iontophoresis, a medical device is used to pass a mild electrical current through water and through the skin’s surface1. Aluminum chloride or anticholinergics can be added to the water to help draw electric current. This non-surgical method is painless and effective. However, it requires long-term, weekly therapy to maintain efficacy. Of note, iontophoresis is contraindicated in patients with pacemakers or implants, or during pregnancy.
Microwave device
Non-invasive microwave technology uses thermal energy to ablate sweat glands. The device is FDA approved for treatment of severe axillary HH. The treatment is performed in a physician’s office with local anesthesia. Patients typically require 1-3 treatments to achieve long-lasting results. There is little to no discomfort during the procedure. Side effects are minor and include underarm swelling, redness, and tenderness lasting for several days. Numbness and tingling can occur in the upper arm or armpit and may last for about 5 weeks1.
Systemic Therapies
There are no systemic agents FDA approved for treatment of HH. Most data for systemic treatment comes from case reports or small case series. Systemic treatment may be more helpful in multifocal hyperhidrosis or hyperhidrosis of spinal cord injury. The two most common prescribed oral agents include Glycopyrrolate and Oxybutynin.
Glycopyrrolate does not cross the blood-brain barrier, resulting in less CNS effects. There is also an oral solution available for pediatric populations. Oxybutynin is safe in children as well. These therapies are cost-effective, but long-term therapy is required.
-
- Adverse effects due to Anticholinergic properties:
- Dry mouth
- Dry eyes
- Constipation
- Blurred vision
- Difficulty with urination
- Adverse effects due to Anticholinergic properties:
There is a risk of hyperthermia in pediatric patients taking systemic anticholinergics. It is important to watch for behavioral changes, as well as drowsiness, dizziness and confusion. Despite the efficacy, most patients discontinue therapy due to anticholinergic side effects.
Other systemic therapies include beta-blockers and Clonidine. Beta-blockers can be used for social phobias/performance anxiety. However, it is contraindicated in patients with bradycardia, AV block, or asthma. Of note, beta-blockers are a common cause of drug-induced psoriasis or exacerbation of psoriasis. Consider other treatment options in patients suffering from both psoriasis and HH. Clonidine has been evaluated in small case series. It is a great medication in treating patients with both hyperhidrosis and flushing.
Minimally invasive approaches
OnabotulinumtoxinA is FDA approved for axillary hyperhidrosis unresponsive to other therapies. It prevents release of acetylcholine from the neuromuscular junction. Injection technique involves a grid pattern (mapped via surgical marker) using a 30-gauge needle. Injection depth is approximately 2 mm and at a 45º angle to the skin surface with bevel side up. Injections are spaced 1-2cm apart for a total of 50 units injected per axilla.

The 2021 ODAC Dermatology, Aesthetic & Surgical Conference took place virtually January 14 – 17, 2021. Below is a current conference coverage recap. Additional articles will be added and available soon.
What’s best for filler injection, needle or cannula?
Source: MDEdge Dermatology
Expert calls for paradigm shift in lab monitoring of some dermatology drugs
Source: MDEdge Dermatology
Next-Generation Therapies and Approaches Will Advance Acne Treatment
Source: DermatologyTimes
Multiple Modalities Help Revise Scars
Source: DermatologyTimes
Click here to view the full poster PDF.
The following poster was presented at the 2021 Virtual ODAC Dermatology, Aesthetic & Surgical Conference.
Authors: Morgan Arnold MD, Elise Weisert BS, Paige Hoyer MD, Janice Wilson MD, Brandon Goodwin MD
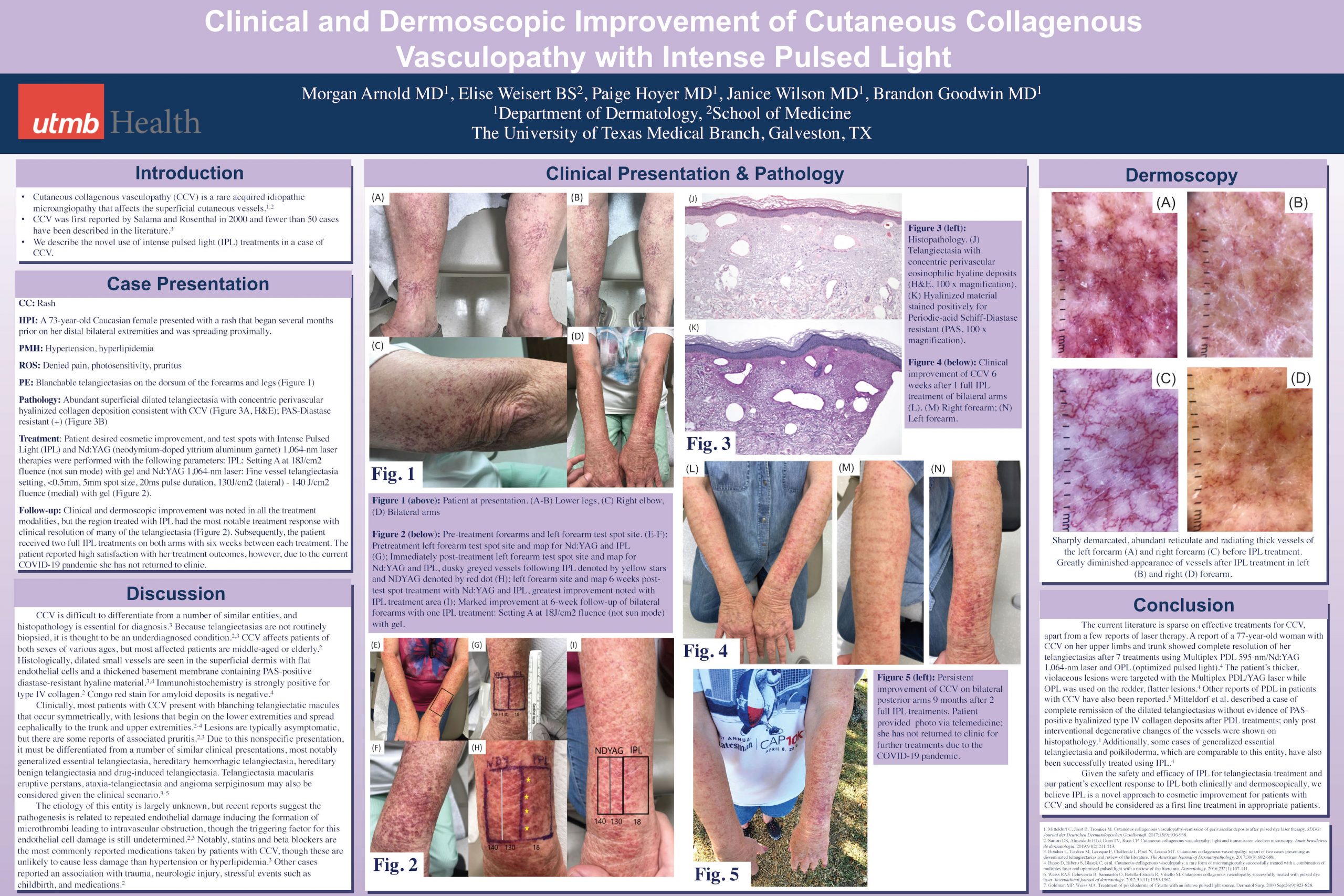
Cutaneous collagenous vasculopathy (CCV) is a rare acquired idiopathic microangiopathy that affects the superficial cutaneous vessels. We describe the use of intense pulsed light (IPL) treatment in a case of CCV resulting in clinical and dermoscopic improvement. A 73-year-old Caucasian female presented with a rash on the arms and legs spreading proximally. Histopathology revealed abundant superficial dilated telangiectasia with concentric perivascular hyalinized collagen deposition and hyalinized material stained positively for Periodic-acid Schiff-Diastase resistant, confirming a diagnosis of CCV. Intense Pulsed Light (IPL) and Nd:YAG (neodymium-doped yttrium aluminum garnet) test spots were performed, with marked improvement with IPL therapy on follow-up. Two subsequent full IPL treatments were performed on patient’s bilateral arms, exhibiting significant clinical and dermoscopic improvement. Though effective treatments for IPL are scarce in the literature, there are reports of treatment with Multiplex PDL 595-nm/Nd:YAG 1,064-nm laser and optimized pulsed light. Furthermore, comparable entities to CCV such as generalized essential telangiectasia and poikiloderma have also been shown to be successfully treated with IPL. We present the use of IPL for successful cosmetic therapy in a patient with CCV.

For most dermatologic conditions, my mantra for treatment tends to be “less is more”. I prefer to use the fewest number of creams, pills, and steps to achieve the best results. However, after watching this year’s ODAC Sneak Peek Inflammatory Diseases Symposium, I may have a new mantra for treating my rosacea patients – “forks, spoons, and knives”.
At the ODAC 2021 Pre-Conference Sneak Peek Inflammatory Diseases Symposium, Dr. Julie Harper, dermatologist at the Dermatology and Skin Care Center of Birmingham and Founding Director of the American Acne and Rosacea Society, built a case for use of combination therapy for rosacea. It is well known that there are four subtypes of rosacea – erythematotelangiectatic, papulopustular, phymatous, and ocular. However, most patients don’t fall into one subtype, but rather, many experience subtype overlap. This is why Dr. Harper believes that clinicians should “start subtyping lesions” and treat all the lesions that are observed, even if that means use of multiple therapeutic agents.
Not only are there multiple targets clinically, but treatment for rosacea can also be targeted on a cellular level. The role of innate immunity including cathelicidins, specifically LL-37, and toll-like receptor 2 induction via demodex has been described in the pathogenesis of rosacea. But did you know that many of the well-known triggers of rosacea such as heat, stress, exercise, and spice have been implicated to affect rosacea on a cellular level? I certainly didn’t! Dr. Harper explained that common triggers of rosacea cause neurogenic inflammation via induction transient receptor potential channels to release substance P and calcitonin gene-related peptide, which cause vasodilation.
Forks, Spoons, and Knives
To better understand therapeutic options, Dr. Harper compares the various treatments to utensils, categorizing them into forks, spoons, or knives. She states that treating rosacea is like eating a meal – you can’t get by with just one utensil. While a spork would be a plausible loophole to this one-utensil-meal challenge, we don’t quite have a spork for treating rosacea (yet!). So what exactly are the utensils that Dr. Harper explained?
Forks are medications that treat papules and pustules, including:
-
- Ivermectin
- Metronidazole
- Azelaic acid
- Sodium sulfacetamide/sulfur
- Modified release doxycycline
Spoons should be utilized for persistent background erythema, such as:
-
- Brimonidine
- Oxymetazoline
Finally, knives are device-based treatments:
-
- Pulsed dye laser
- KTP
- IPL
- Electrosurgery
Why Combination Therapy?
Before showing us the evidence that combination therapy works, Dr. Harper described the goals of combination therapy:
-
- Achieve clear skin
- Achieve treatment goals more quickly
- Maximize remission periods
- Minimizing burden of disease
First, she reviewed a JDD article (Fowler J. J Drugs Dermatol. 2007;6(6)641-646) that combined two forks: modified release doxycycline and metronidazole gel. This 16-week study compared combination modified-release doxycycline with metronidazole 1% gel to metronidazole 1% gel with placebo. Metronidazole was discontinued at week 12 and doxycycline or placebo continued through week 16. At week 4, patients who received combination therapy had greater reduction of inflammatory lesion counts and were generally better faster. Then after week 12, those who continued on modified release doxycycline had better sustained results.
Next, Dr. Harper reviewed another JDD study (Stein Gold L, Papp K, et al. J Drugs Dermatol. 2017;16(9):909-916) that combined a fork and a spoon – ivermectin and brimonidine – to treat both moderate to severe erythematotelangiectatic and papulopustular rosacea. Three groups were identified in this study – one receiving vehicle only, one receiving ivermectin for 12 weeks with addition of bromonidine at week 4, and one receiving both ivermectin and brimonidine for 12 weeks. The most striking result from this study was that those who used combination therapy for 12 weeks had double the rate of improvement of redness in the three hours after brimonidine use. Dr. Harper explained that despite the use of a really good fork (ivermectin), there was still enough residual redness that the spoon (brimonidine) could make a meaningful impact. Thus, targeting inflammation and pustules alone will not lessen background erythema.

This year, due to the global pandemic, we are hosting ODAC Dermatology, Aesthetic and Surgical conference, virtually. ODAC is committed to providing high-quality education in an engaging virtual format with top speakers and important topics.
With nearly 1,000 US attendees registered, ODAC will provide a unique virtual experience that will continue to drive dermatology education and networking forward.
We appreciate all the effort that it takes to change direction and to make such an important and impactful event happen. We are deeply grateful to our sponsors, faculty and attendees.
Please take a moment to acknowledge and thank our gracious supporters by visiting their sponsor page in ODAC Virtual app.
Platinum and Featured Sponsors
Please see the resources below to discover our data and explore our resources in our sponsor page.

Cassiopea, Inc. is a specialty pharmaceutical company developing and commercializing prescription drugs with novel mechanisms of action to address long-standing and essential dermatological conditions such as acne, androgenetic alopecia and genital warts. Learn more about our company at www.cassiopea.com.
Our commitment to dermatology is built around a senior leadership team with deep roots and experience in the specialty. The management team is focused on providing unique treatment options, and to drive continued research and development in Dermatology, moving us to the forefront of the U.S. market.
We are excited to support dermatology healthcare professionals by contributing to the ODAC 2021 Conference.
Galderma, the world’s largest independent global dermatology company, was created in 1981 and is now present in over 100 countries with an extensive product portfolio of prescription medicines, aesthetics solutions and consumer care products. The company partners with health care practitioners around the world to meet the skin health needs of people throughout their lifetime. Galderma is a leader in research and development of scientifically-defined and medically-proven solutions for the skin. For more information, please visit www.galderma.com/us.
Incyte is a global biopharmaceutical company that is focused on finding solutions for serious unmet medical needs through the discovery, development and commercialization of novel medicines.
Since 2002, Incyte has remained committed to the relentless pursuit of science that can improve the lives of patients, make a difference in healthcare and build sustainable value for our stakeholders. The Company is advancing a diversified portfolio of clinical candidates across Oncology and Inflammation & Autoimmunity. Our research and development efforts in Dermatology are focused on a number of immune-mediated dermatologic conditions with a high unmet medical need, including atopic dermatitis, vitiligo, and hidradenitis suppurativa.
Headquartered in Wilmington, Delaware, Incyte has operations in North America, Europe and Asia.
For more information, visit Incyte.com and follow @Incyte.
Visit the Incyte Dermatology Virtual Booth.
At Aveeno®, groundbreaking science transforms nature’s ingredients into clinically proven products that help soothe, strengthen, and restore the moisture barrier. Our oat, soy, and feverfew ingredients—derived from nature and uniquely formulated to optimize skin health and beauty—are supported by 75 years of published data, demonstrating the scientific rigor behind every result. Visit AveenoMD.com to learn about our new products, plus access clinical resources, patient resources, and product samples.
The #1 dermatologist recommended skincare brand continually elevates the science of skincare, delivering new products and innovations that support your treatment guidance. Visit NeutrogenaMD.com to learn what’s new, plus access clinical resources, patient resources, and product samples. NeutrogenaMD.com – dedicated to dermatology professionals.
Our brands are dedicated to dermatology with offerings in professional skincare and cosmetics. Each brand is created by dermatologists or works in partnership with dermatologists to meet the health and safety standards for patients. Please visit the L’Oréal booth.
Novartis is a leading global medicines company, using innovative science and digital technologies to create treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis products reach more than 800 million people globally and we are finding innovative ways to expand access to our latest treatments. About 109,000 people of more than 145 nationalities work at Novartis around the world. Find out more at www.novartis.com.
We hope you will take a moment to visit the COSENTYX® (secukinumab) Virtual Booth Experience to learn more about the Complete Cosentyx Approach.

Ortho Dermatologics is a specialty pharmaceutical business with the number one prescribed acne franchise and a portfolio of dermatology treatments in additional therapeutic areas including atopic dermatitis, fungal infections and psoriasis. Led by a team with deep experience in dermatology, we are dedicated to building meaningful connections with healthcare providers and their patients. Our ongoing commitment to the dermatology community shows through our pipeline of novel compounds and focused philanthropic activity here and around the globe.

Regeneron is a leading biotechnology company that invents medicines for people with serious diseases. Founded and led for over 30 years by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to seven FDA-approved treatments and numerous product candidates in development, all of which were homegrown in our laboratories.
Sanofi is dedicated to supporting people through their health challenges. We are a global biopharmaceutical company focused on human health. We stand by the few who suffer from rare diseases and the millions with long-term chronic conditions. With more than 100,000 people in 100 countries, Sanofi is transforming scientific innovation around the globe.
Please click the link below to visit our virtual booth!
https://www.dupixenthcp.com/virtualcongress
At UCB, we come together every day to work, laser-focused, on a simple question: How will this create value for people living with severe diseases? Patient value is not just what we say, but how we how we live. It is our culture of care, embodied by our patient value strategy. That’s because how we do business – from discovery to development to delivery – has been transformed and redesigned around the patient and their individual experience. Patients are at the heart of everything we do, inspiring us, driving our scientific discovery, and leading us to rethink the patient experience. By fulfilling our commitment, driving innovation, and providing patients a meaningful experience, more impactful solutions are on the horizon.
With a team of approximately 7,500 employees and operations in nearly 40 countries, we are a global biopharmaceutical company investing more than a quarter of our revenue in cutting-edge scientific research to meet unmet patient needs. Global headquarters are in Brussels, Belgium, with U.S. headquarters in Atlanta, Georgia. Additional U.S. UCB sites include global clinical development in Raleigh, North Carolina, research supporting UCB’s pipeline in Boston, Massachusetts (Bedford and Cambridge), Seattle, Washington, and Durham, North Carolina as part of our acquisition of Element Genomics, as well as an office in Washington, D.C.
CLICK to ENTER Virtual Experience
Sheila Barbarino, MD, FACS, FAAO, FAACS
Owner
Institution Barbarino Surgical Arts
Austin, TX & El Segundo, CA
ODAC
Learn what integrated skin care is and how to incorporate skincare to compliment your in-office procedures. All US-based practitioners that attend the full webinar, will receive a 30 mL CE Ferulic!
Date: Tuesday, December 8, 2020
Time: 7:00 – 8:00 PM ET
Faculty: Sheila Barbarino, MD, FACS, FAAO, FAACS